Headline
JUST IN : COVID-19 Vaccine Gets NAFDAC Approval For Use In Nigeria
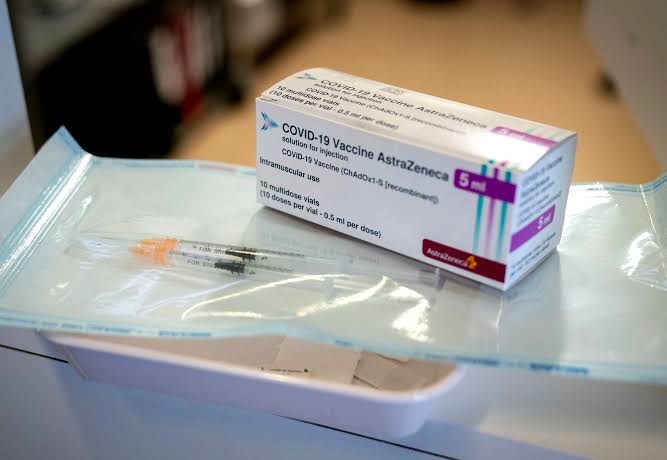
COVID-19 Vaccine Gets NAFDAC Approval For Use In Nigeria.
OnyxNews Nigeria reports that the National Agency for Food and Drug Administration and Control, NAFDAC, has approved Oxford/AstraZeneca COVID-19 vaccine for use in Nigeria.
NAFDAC Director-General, Dr. Mojisola Adeyeye made it known on Thursday at a press briefing in Abuja.
She explained that the vaccine can be stored at 2 to 8-degree centigrade.
AstraZeneca vaccine was recently approved by the World Health Organisation, WHO.
The Oxford/AstraZeneca vaccine, also known as ChAdOx1 nCoV-19, or AZD1222, is a viral vector vaccine.
However, the NAFDAC boss said the body got the dossier of the vaccine a week ago and its safety committee went to work immediately to evaluate its safety and efficacy for Nigerians.
She said there are three additional vaccines undergoing evaluation, but the evaluation on Astrazeneca shows that the vaccine is effective against the UK variant of the virus which has been reported in Nigeria.
READ Why Nigeria Was Disqualified From Global Vaccine Bid By WHO
Dr Adeyeye revealed that the South African variant has not been reported in Nigeria, adding that the agency has over 30 herbal medicine undergoing review for listing.
-

 Brands and Marketing5 days ago
Brands and Marketing5 days agoUPDATED: See Naira Exchange Rate Against Dollar
-

 Headline1 week ago
Headline1 week agoSenate Sets Date For NAFDAC Enforce Ban On Sachet Alcohol— See Date
-

 Headline1 week ago
Headline1 week agoUCL: Osimhen Shortlisted For Player Of The Week Award, See Other Nominees
-

 Headline3 days ago
Headline3 days agoFIFA W’CUP 2026: Messi Reveals Condition To Play For Argentina
-

 Headline1 week ago
Headline1 week agoJUST IN: White House Guest Faints Beside U.S Donald Trump, See Details
-

 Headline4 days ago
Headline4 days agoChina Orders Gay Dating Apps Be Removed From Stores
-

 Headline4 days ago
Headline4 days agoBREAKING: Court Bars PDP From Holding National Convention
-

 Headline3 days ago
Headline3 days agoVIDEO: Tension In Abuja As Soldiers Block Wike From Accessing Estate Land
-

 Headline1 day ago
Headline1 day agoFIFA W’CUP: Super Eagles Storm Into Playoff Final After 4–1 Extra-Time Win Over Gabon
-

 Headline3 hours ago
Headline3 hours agoMajor Glo Network Outage Cripples Services Across Abuja, Northern States— Reports






